KAIST found burning ice in oceanic clay rich sediments
Have you ever heard of flammable ice? If not, this is your chance to know about one of the most exotic natural phenomena where methane gets enclosed inside a water crystal that looks similar to an ice cube. Also known as Methane clathrate, this formation can be found in the sediments of ocean floors of the Earth. Recently a group of scientists from the Korea Advanced Institute of Science and Technology has identified the natural abundance of gas hydrates created in oceans. Not just that, this identification has raised questions regarding the formation of such burning ice.
A team led by Professor Tae-Hyuk Kwo, from the Department of Civil & Environmental Engineering has discovered clay minerals on the oceanic clay-rich sedimentary deposits that in turn instigate the formation of gas hydrates. Like already explained, these gas hydrates are nothing but the encapsulated methane molecules inside the cage of hydrogen-bonded water molecules. And these deposits attract attention from the people interested in alternative energy.
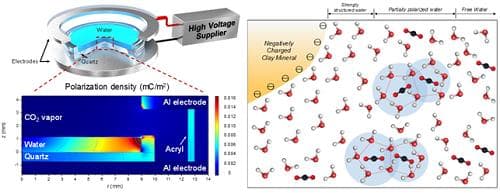
Formation of Gas Hydrates using water molecules
Till now, it was believed that these gas hydrates are limited to clay sedimentary deposits however the sudden discovery of gas hydrates in oceanic clay-rich sedimentary deposits changed the course of its existence. The surfaces of natural clay minerals possess negative charges and it creates a physicochemical interaction between clay and water. This explains why gas hydrates naturally get formed in the clay-rich sedimentary deposits.
The experimental verification, however, is much complicated since the cations present in clay particles balance out the clay surface charges. So when mixed with water, clay particles release cations that complicate the experimental findings. This issue was resolved by using polarized water molecules with an electric field and then monitoring the induction times required for the molecules to form gas hydrates.
The result showed that 10 kV/m of electric field proffered gas hydrate nucleation under specific conditions which included partial breakage of the hydrogen-bonded water clusters and the lowered thermal energy of water molecules. According to Professor Kwon, this research will enable future projects to commercially produce methane via natural gas hydrate deposits. The research has been published in the Environmental Science and Technology journal.
Source: #-Link-Snipped-#
A team led by Professor Tae-Hyuk Kwo, from the Department of Civil & Environmental Engineering has discovered clay minerals on the oceanic clay-rich sedimentary deposits that in turn instigate the formation of gas hydrates. Like already explained, these gas hydrates are nothing but the encapsulated methane molecules inside the cage of hydrogen-bonded water molecules. And these deposits attract attention from the people interested in alternative energy.

Formation of Gas Hydrates using water molecules
Till now, it was believed that these gas hydrates are limited to clay sedimentary deposits however the sudden discovery of gas hydrates in oceanic clay-rich sedimentary deposits changed the course of its existence. The surfaces of natural clay minerals possess negative charges and it creates a physicochemical interaction between clay and water. This explains why gas hydrates naturally get formed in the clay-rich sedimentary deposits.
The experimental verification, however, is much complicated since the cations present in clay particles balance out the clay surface charges. So when mixed with water, clay particles release cations that complicate the experimental findings. This issue was resolved by using polarized water molecules with an electric field and then monitoring the induction times required for the molecules to form gas hydrates.
The result showed that 10 kV/m of electric field proffered gas hydrate nucleation under specific conditions which included partial breakage of the hydrogen-bonded water clusters and the lowered thermal energy of water molecules. According to Professor Kwon, this research will enable future projects to commercially produce methane via natural gas hydrate deposits. The research has been published in the Environmental Science and Technology journal.
Source: #-Link-Snipped-#
0
