Binuclear Copper Complex Converts Carbon Dioxide To Organic Compound- Oxalate
The rapid increase in the level of pollutants like carbon dioxide (CO2) in the environment is a growing concern and many researches are going on in this field in order to reduce its atmospheric presence. One such research was carried out by the researchers of Louisiana State University (LSU) and their work was published in the Journal Nature Communications on December,19. The research team has discovered a binuclear copper complex that converts carbon dioxide to oxalate (C2O42−) by applying a three-step reaction sequence. In other words, this new method converts CO2 into a useful organic compound.
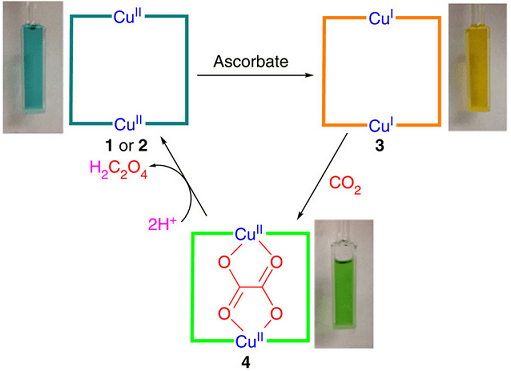
Diagrammatic representation of conversion of CO2 to oxalate
The team led by Andrew Maverick, Professor of Chemistry and acting associate dean in the LSU College of Science, performed three-step reaction sequence with help of a mild reducing agent. The process starts with the reduction of cyclic copper (II) complex to copper (I) in a solution. The reduction is done electrochemically or using sodium ascorbate (vitamin C). After that, the CO2 present in the air is fixed into oxalate as the reduced complex selectively reacts with it. Then, the bridging of oxalate ion between two copper atoms takes place, which leads to its release as oxalic acid after treating with mineral acids. This leads to the regeneration of original copper complex as oxalate and the completion of the process.
Developing a compound that would react with CO2 was a herculean task as CO2 does not react with any compound easily. The team formed more than 50 distinct compounds before creating copper complex. According to Maverick, even highly energetic molecules many times do not react with CO2 and to find a compound that can convert CO2 into something with a little more stored energy was of great significance while performing the research.
The major drawback of this research is that the compound takes four to five days to react. Therefore, the team is currently focusing on this factor. They are also trying to find other compounds and catalysts that will speed the conversion process.
Source: #-Link-Snipped-#| #-Link-Snipped-#
Diagrammatic representation of conversion of CO2 to oxalate
The team led by Andrew Maverick, Professor of Chemistry and acting associate dean in the LSU College of Science, performed three-step reaction sequence with help of a mild reducing agent. The process starts with the reduction of cyclic copper (II) complex to copper (I) in a solution. The reduction is done electrochemically or using sodium ascorbate (vitamin C). After that, the CO2 present in the air is fixed into oxalate as the reduced complex selectively reacts with it. Then, the bridging of oxalate ion between two copper atoms takes place, which leads to its release as oxalic acid after treating with mineral acids. This leads to the regeneration of original copper complex as oxalate and the completion of the process.
Developing a compound that would react with CO2 was a herculean task as CO2 does not react with any compound easily. The team formed more than 50 distinct compounds before creating copper complex. According to Maverick, even highly energetic molecules many times do not react with CO2 and to find a compound that can convert CO2 into something with a little more stored energy was of great significance while performing the research.
The major drawback of this research is that the compound takes four to five days to react. Therefore, the team is currently focusing on this factor. They are also trying to find other compounds and catalysts that will speed the conversion process.
Source: #-Link-Snipped-#| #-Link-Snipped-#
0
