University of Illinois Engineers Develop Method To Make Sea Water Drinkable
The troubles with the traditional reverse osmosis process are many. For instance, it requires a lot of power, is very expensive and the membranes fail due to clogging.
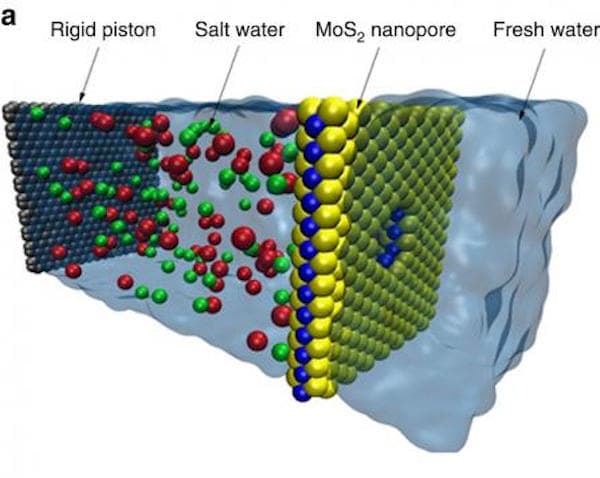
The Illinois team of researchers found that MoS2 nanopores were much better than graphene membranes at water desalination, thanks to its chemical properties, thinness and pore geometry. MOS2 has molybdenum in the center for attracting water, then there's sulfur on the side to push it away and therefore water gets easily passed through it.
Moreover, with its natural thinness, the MoS2 single layer sheets don't need much energy and therefore is great for reducing operating costs. Since it is a robust material, it's great at withstanding high water volume pressure as well.
There's a lot of water on this planet, but most of it is not drinkable. If such an efficient, low-cost solution to purify sea water is developed, we could all solve the big water crisis.
What are your thoughts about sea water desalination techniques? Share with us in comments below.
Source: #-Link-Snipped-#
