Metal Carbides Can Lower The Cost Of Pricey Catalysts – MIT Research
Catalyst- a common expression which appears in almost every chapter of a chemistry textbook holds a key role in deciding the fate of a chemical reaction. Be it organic or inorganic, catalysts are used everywhere. It is basically a chemical element or a compound that either accelerates or retards the rate of a chemical reaction without participating in it. However, they are often very pricey as most of them contains one or more noble elements like platinum, palladium etc. The supply of these noble- rare elements are limited which raises the price bar of these materials.
Recently, researchers at MIT found out a technique to utilize a minimal amount of these rare noble elements to carry out their catalytic properties at a lesser price. They tried to incorporate the science of nanotechnology behind it. They tried to provide a coating of a noble metal over a tiny particle which is more abundant and inexpensive in nature. By using metal carbides as the core, they achieved a promising result.
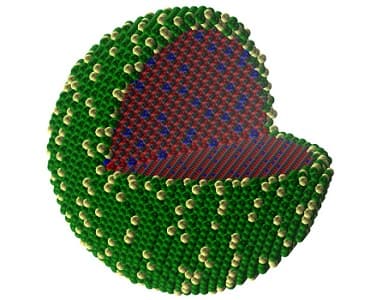
A Simulation of the Core-Shell Structure
As only the surface of the catalytic particles are involved in a chemical reaction, replacing their core by a metal carbide can reduce the consumption of the noble elements. The work has been carried out by the students Sean Hunt, Maria Milina, Christopher Hendon, and Associate Professor Yuriy Román-Leshkov of the Department of Chemical Engineering at MIT and appeared in the Journal, Science this week.
For the past few years, many researchers were trying to carry out such activity, but were unable to get a breakthrough. The reason behind it was either the noble elements used to form alloys with the core particle or doesn’t interact with it causing instability. Further, transition metal carbides are very difficult to engineer into nano particles by keeping their property as desired. The key breakthrough was achieved by using a silica template along with the proposed materials. The silica template keeps the shell and core close during high temperature interactions and thus allow them to bind together. Silica is removed afterwards via acidic treatment.
The importance of this discovery is that not only metal carbides but materials like titanium, tungsten can also be used as a core-substitute to enhance the catalytic effect of the existing materials. These types of catalysts are often plagued by poisoning and thus after few reaction cycles, their efficiency gets reduced. Using this newer technique provides catalytic materials better efficiency and supports more reaction cycle than they were supposed to.
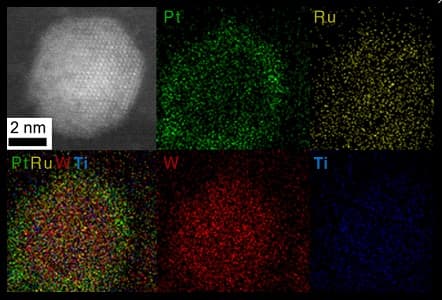
Displaying the Core-Shell concentration on nanoscale
Catalysts are used in almost every field- from vehicle emission to electrolyzers to fuel cells to industries. Incorporating this newer technology will not only speed up the manufacturing process but will also reduce the price bar of these costly materials.
Source- New nanoparticle catalysts could reduce need for precious metals | MIT News | Massachusetts Institute of Technology | #-Link-Snipped-#
Recently, researchers at MIT found out a technique to utilize a minimal amount of these rare noble elements to carry out their catalytic properties at a lesser price. They tried to incorporate the science of nanotechnology behind it. They tried to provide a coating of a noble metal over a tiny particle which is more abundant and inexpensive in nature. By using metal carbides as the core, they achieved a promising result.

A Simulation of the Core-Shell Structure
As only the surface of the catalytic particles are involved in a chemical reaction, replacing their core by a metal carbide can reduce the consumption of the noble elements. The work has been carried out by the students Sean Hunt, Maria Milina, Christopher Hendon, and Associate Professor Yuriy Román-Leshkov of the Department of Chemical Engineering at MIT and appeared in the Journal, Science this week.
For the past few years, many researchers were trying to carry out such activity, but were unable to get a breakthrough. The reason behind it was either the noble elements used to form alloys with the core particle or doesn’t interact with it causing instability. Further, transition metal carbides are very difficult to engineer into nano particles by keeping their property as desired. The key breakthrough was achieved by using a silica template along with the proposed materials. The silica template keeps the shell and core close during high temperature interactions and thus allow them to bind together. Silica is removed afterwards via acidic treatment.
The importance of this discovery is that not only metal carbides but materials like titanium, tungsten can also be used as a core-substitute to enhance the catalytic effect of the existing materials. These types of catalysts are often plagued by poisoning and thus after few reaction cycles, their efficiency gets reduced. Using this newer technique provides catalytic materials better efficiency and supports more reaction cycle than they were supposed to.

Displaying the Core-Shell concentration on nanoscale
Catalysts are used in almost every field- from vehicle emission to electrolyzers to fuel cells to industries. Incorporating this newer technology will not only speed up the manufacturing process but will also reduce the price bar of these costly materials.
Source- New nanoparticle catalysts could reduce need for precious metals | MIT News | Massachusetts Institute of Technology | #-Link-Snipped-#
Replies
You are reading an archived discussion.
Related Posts
Adam Savage from Mythbusters once said that the difference between screwing around and science is writing stuff down. Google seems to have noted things down and is now helping you...
In December 2013, Akshat Singhal, who is pursuing Msc Physics and B.E(Hons.) in Civil Engineering from BITS Pilani, started Legistify (previously known as GetLegal), as an online forum where you...
Omate, the Chinese OEM, has unveiled a new smartwatch - Omate S3, in an event held in Monaco. The company which designs and makes hardware and software for its wearable...
Wearables are the next big thing in technology but the creations we have seen so far implement electronics from the present generation which cannot bend or contour with our body...
