Physicists Synthesize 'Artificial Graphene' By Replacing Carbon Atoms With Transition Metals
A group of scientists from the University of Arkansas has developed an artificial material that is said to have a structure similar to that of <a href="https://www.crazyengineers.com/threads/graphene-sponge-paves-way-for-propulsion-system-in-solar-spacecrafts.80934">'Graphene Sponge' Paves Way For Propulsion System In Solar Spacecrafts</a>. Instead of Carbon, the new material consists of transition metal atoms arranged in a honeycomb-shaped lattice. The research was funded jointly by the Gordon and Betty Moore Foundation and the Chinese Academy of Science.
Discovered in 2004, Graphene is a one-atom-thick sheet of graphite- an allotrope of Carbon. Contemporary use of graphene can be seen in electronics, biological engineering, filtration, in lightweight and strong composite materials, photovoltaics and in energy storage. Graphene transistors are potentially faster and more heat tolerant than today’s silicon transistors and may be used as the future constituent of next generation computer chips.
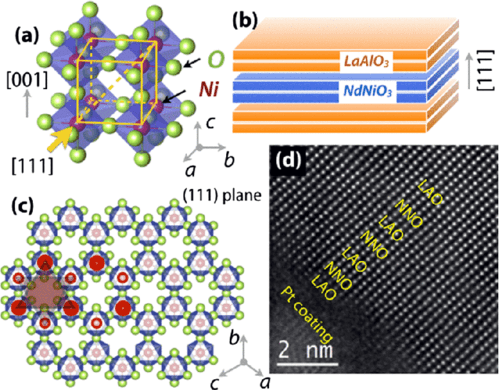
Artificial Graphene like structure with sandwiched transient metal atoms
Srimanta Middey, a postdoctoral research associate at the University of Arkansas along with quantum materials investigator, Jack Chakhalian have jointly developed a Rare-Earth-based nickelate structure similar to Graphene that might have a significant impact on the printing industry. The research group also included University of Arkansas postdoctoral research associates Michael Kareev and Yanwei Cao, doctoral student Xiaoran Liu and recent doctoral graduate Derek Meyers.
Researchers replaced the carbon atoms in the crystalline structure of graphene by experimentally incorporating transition elements in them. Surprisingly, the foreign elements bonded successfully with the atoms and gave rise to a stable structure.
The research findings were reported this week in Physical Review Letters, a journal of the American Physical Society titled “Mott Electrons in an Artificial Graphene-like Crystal of Rare Earth Nickelateâ€.
Watch the next big thing here:
Source: <a href="https://news.uark.edu/articles/33478/physicists-create-artificial-graphene-" target="_blank" rel="nofollow noopener noreferrer">Physicists Create Artificial 'Graphene' | University of Arkansas</a> | <a href="https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.116.056801" target="_blank" rel="nofollow noopener noreferrer">403 Forbidden</a>
Discovered in 2004, Graphene is a one-atom-thick sheet of graphite- an allotrope of Carbon. Contemporary use of graphene can be seen in electronics, biological engineering, filtration, in lightweight and strong composite materials, photovoltaics and in energy storage. Graphene transistors are potentially faster and more heat tolerant than today’s silicon transistors and may be used as the future constituent of next generation computer chips.

Artificial Graphene like structure with sandwiched transient metal atoms
Researchers replaced the carbon atoms in the crystalline structure of graphene by experimentally incorporating transition elements in them. Surprisingly, the foreign elements bonded successfully with the atoms and gave rise to a stable structure.
The research findings were reported this week in Physical Review Letters, a journal of the American Physical Society titled “Mott Electrons in an Artificial Graphene-like Crystal of Rare Earth Nickelateâ€.
Watch the next big thing here:
Source: <a href="https://news.uark.edu/articles/33478/physicists-create-artificial-graphene-" target="_blank" rel="nofollow noopener noreferrer">Physicists Create Artificial 'Graphene' | University of Arkansas</a> | <a href="https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.116.056801" target="_blank" rel="nofollow noopener noreferrer">403 Forbidden</a>
0

