Intrinsic and Extrinsic semiconductors
Intrinsic and Extrinsic Semiconductors
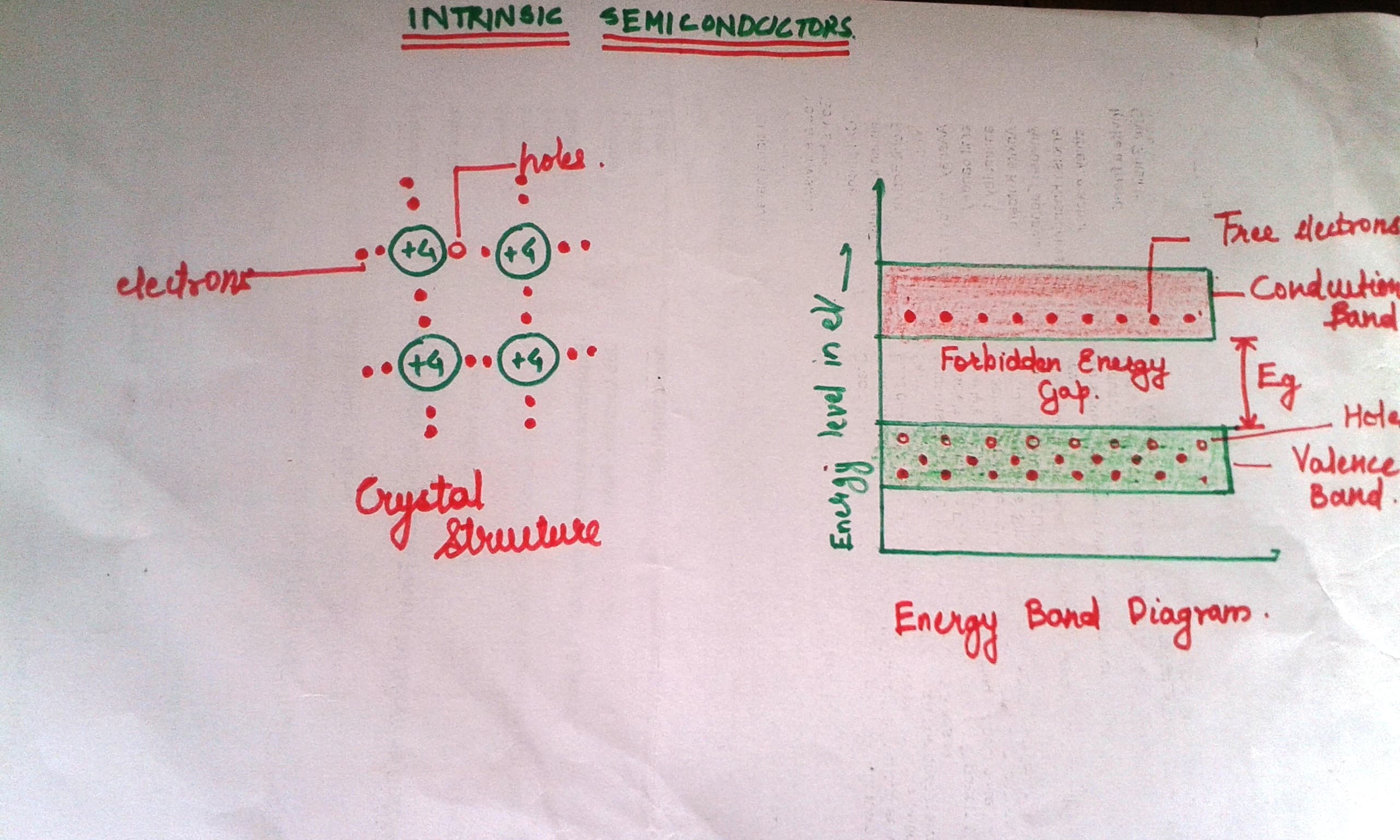
Intrinsic Semiconductors:
1. Semiconductors material in extremely pure form is called intrinsic semiconductor.
2. Free electrons and holes are generated in pairs. Thus concentration of holes is equal to that of electrons.
3. At absolute zero temperature (-273°C), the intrinsic semiconductor, all the electrons are tightly held by their atoms. With increase in temperature the electrons break in through the atoms, and move from valence band to conduction.
4. Temperature increase à electrons - holes pair generation increase àconductivity of intrinsic conductor increase à resistivity decrease.
5. Thus semiconductors have negative temperature coefficient.
Extrinsic Semiconductors:
1. Addition of impurity to pure semiconductor results into extrinsic semiconductor. It is known as doping.
2. The material that is added to it is called doped material. Germanium or silicon is tetravalent. (4 electrons in the outermost orbit). So either a pentavalent or trivalent impurity can be added to it.
3. It has high conductivity as compared to that of intrinsic semiconductor even at room temperature .
4. According to the added impurity they are classified as:
a. Donor type or N-type
b. Accepter type or P-type
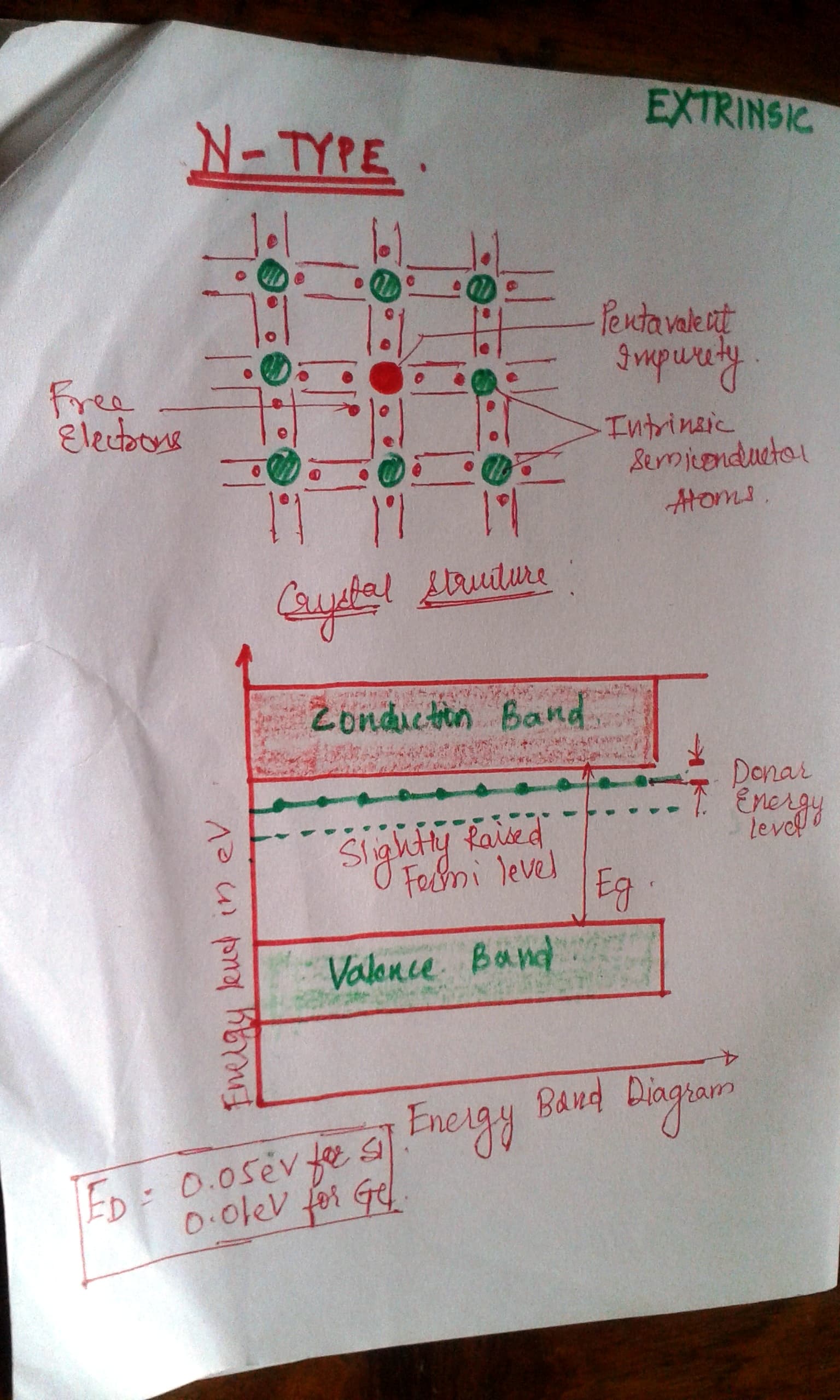
A. N-type Extrinsic Semiconductors:
1. Pentavalent impurity is added.
2. Arsenic, phosphorus
3. So one electrons is left enters into the conduction band as a free electron. So negative charge. Thus N-type
4. Number of holes is small as compared to the number of electrons.
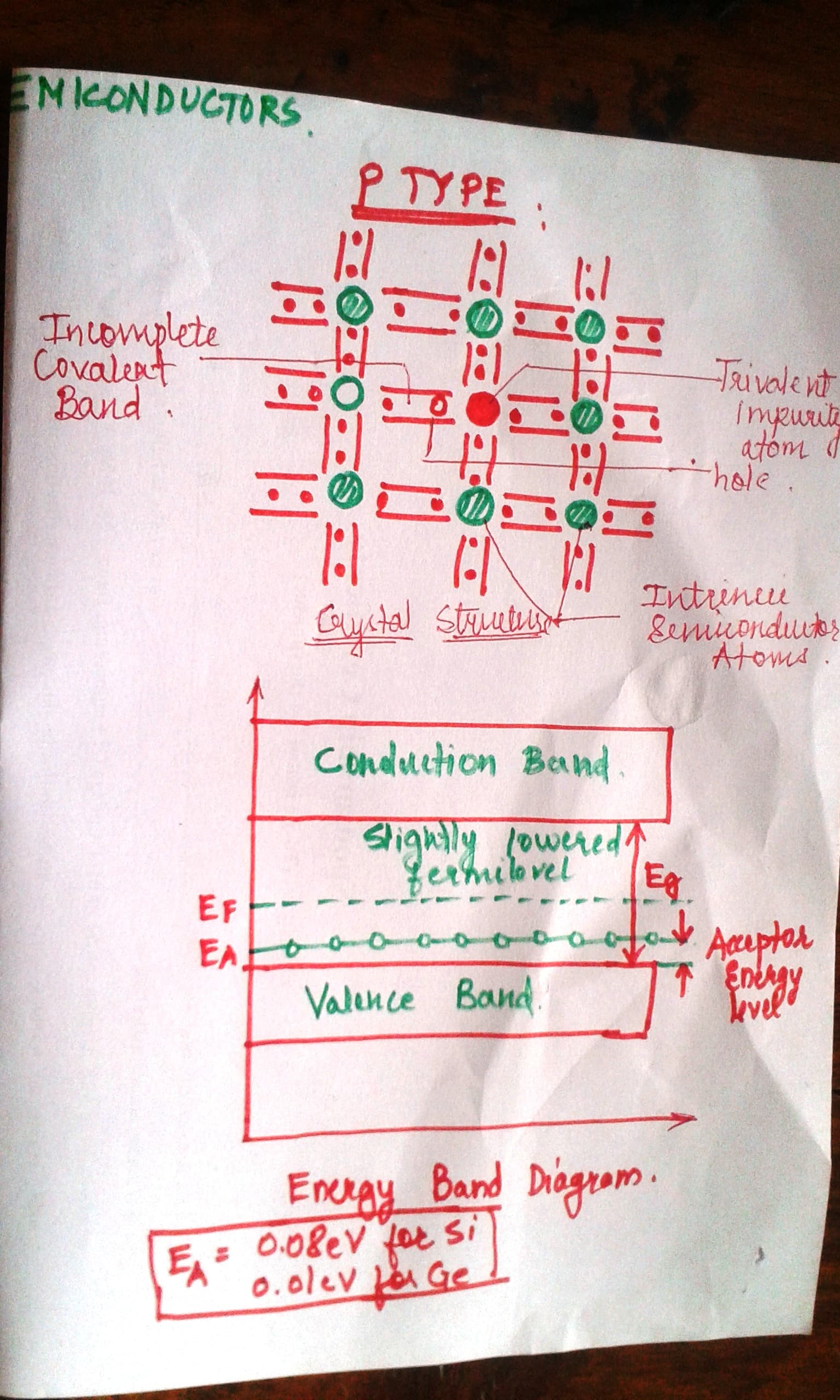
B. P-type Extrinsic Semiconductors:
1. Trivalent impurity
2. Boron, alluminium etc
3. So three electrons get combined and one hole is left. Thus positive charge. Therefore P-type.
4. Number of electrons is small as compared to number of electrons.
Q. There are many semiconducting materials, they why mostly Si and Ge is used?
-> because the energy require to break the covalent bonds of Ge and Si is small as compared to other semiconductors. i.e Ge=0.72 eV and Si=1.12 ev.
-> they have same crystal structure.
Intrinsic Semiconductors:
1. Semiconductors material in extremely pure form is called intrinsic semiconductor.
2. Free electrons and holes are generated in pairs. Thus concentration of holes is equal to that of electrons.
3. At absolute zero temperature (-273°C), the intrinsic semiconductor, all the electrons are tightly held by their atoms. With increase in temperature the electrons break in through the atoms, and move from valence band to conduction.
4. Temperature increase à electrons - holes pair generation increase àconductivity of intrinsic conductor increase à resistivity decrease.
5. Thus semiconductors have negative temperature coefficient.

Extrinsic Semiconductors:
1. Addition of impurity to pure semiconductor results into extrinsic semiconductor. It is known as doping.
2. The material that is added to it is called doped material. Germanium or silicon is tetravalent. (4 electrons in the outermost orbit). So either a pentavalent or trivalent impurity can be added to it.
3. It has high conductivity as compared to that of intrinsic semiconductor even at room temperature .
4. According to the added impurity they are classified as:
a. Donor type or N-type
b. Accepter type or P-type
A. N-type Extrinsic Semiconductors:
1. Pentavalent impurity is added.
2. Arsenic, phosphorus
3. So one electrons is left enters into the conduction band as a free electron. So negative charge. Thus N-type
4. Number of holes is small as compared to the number of electrons.

B. P-type Extrinsic Semiconductors:
1. Trivalent impurity
2. Boron, alluminium etc
3. So three electrons get combined and one hole is left. Thus positive charge. Therefore P-type.
4. Number of electrons is small as compared to number of electrons.

Q. There are many semiconducting materials, they why mostly Si and Ge is used?
-> because the energy require to break the covalent bonds of Ge and Si is small as compared to other semiconductors. i.e Ge=0.72 eV and Si=1.12 ev.
-> they have same crystal structure.
0
