Next-Gen Lithium-Ion Batteries Are Inspired From Pomegranate Design
A group of researchers from Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory have come up with an idea of designing an electrode inspired from pomegranate. Just like the seeds inside the fruit, the electrode will be made up of clusters of silicon nanoparticles in a tough carbon rind. This move comes as a solution to the problems faced by the next generation of lithium-ion batteries, which are supposed to be smaller, lighter and more powerful than ever before. After conducting a series of tests on these pomegranate-inspired batteries, Yi Cui, an associate professor at Stanford and SLAC who led the research has shared that even after 1000 cycles of charging and discharging, the anode works at 97% capacity. This just goes to say that it's just a matter of time till we see these batteries being manufactured for commercial use.
In the past, we have seen research work on <a href="https://www.crazyengineers.com/threads/lithium-ion-car-batteries-made-powerful-and-cheaper-by-nanostructures.64044">Lithium Ion Car Batteries Made Powerful And Cheaper By Nanostructures</a> batteries and even the concept of <a href="https://www.crazyengineers.com/threads/lithium-air-battery-stores-4x-as-much-energy-per-pound-as-lithium-ion-batteries.67950">Lithium-Air Battery Stores 4x As Much Energy Per Pound As Lithium Ion Batteries</a>. The pomegranate inspired batteries are unique in more than one way. The anode stores the energy when a battery charges. Use of Silicon anodes instead of graphite ones that are currently used, would mean that the batteries stores 10 times more charge. A major limitation with this has been that silicon is brittle and it swells and falls apart during battery charging, and it reacts with the battery’s electrolyte to form gunk that coats the anode and degrades its performance.

As a solution to this, Cui and his team have been working for the past 8 years, on the use of silicon nanowires or nanoparticles that are too small to break into even smaller bits. They encased these nanoparticles in, what they call, carbon “yolk shells†that give them room to swell and shrink during charging. Taking this research a step forward and drawing inspiration from the chemical industry, the team used a microemulsion technique to gather silicon yolk shells into clusters, and coated each cluster with a second, thicker layer of carbon. These carbon rinds hold the pomegranate clusters together and provide a sturdy highway for electrical currents. And because every pomegranate cluster covers just one-tenth the surface area of the individual particles inside it, a much smaller area is exposed to the electrolyte, thereby reducing the amount of gunk that forms to a manageable level.
Though the tests they have performed on the pomegranate batteries have shown results that are at par with other commercially available batteries, in order to begin mass production of this next-gen product, the researchers need to work on finding a cheaper source of silicon nanoparticles and simplifying the overall process. One of the researchers thinks that rice husks is one of the cheap alternative available today in bulk and it can be transformed into pure silicon nanoparticles relatively easily.
What do you think about the next generation of pomegranate inspired batteries being designed? Share your thoughts with us in comments below.
Source: #-Link-Snipped-#
In the past, we have seen research work on <a href="https://www.crazyengineers.com/threads/lithium-ion-car-batteries-made-powerful-and-cheaper-by-nanostructures.64044">Lithium Ion Car Batteries Made Powerful And Cheaper By Nanostructures</a> batteries and even the concept of <a href="https://www.crazyengineers.com/threads/lithium-air-battery-stores-4x-as-much-energy-per-pound-as-lithium-ion-batteries.67950">Lithium-Air Battery Stores 4x As Much Energy Per Pound As Lithium Ion Batteries</a>. The pomegranate inspired batteries are unique in more than one way. The anode stores the energy when a battery charges. Use of Silicon anodes instead of graphite ones that are currently used, would mean that the batteries stores 10 times more charge. A major limitation with this has been that silicon is brittle and it swells and falls apart during battery charging, and it reacts with the battery’s electrolyte to form gunk that coats the anode and degrades its performance.

As a solution to this, Cui and his team have been working for the past 8 years, on the use of silicon nanowires or nanoparticles that are too small to break into even smaller bits. They encased these nanoparticles in, what they call, carbon “yolk shells†that give them room to swell and shrink during charging. Taking this research a step forward and drawing inspiration from the chemical industry, the team used a microemulsion technique to gather silicon yolk shells into clusters, and coated each cluster with a second, thicker layer of carbon. These carbon rinds hold the pomegranate clusters together and provide a sturdy highway for electrical currents. And because every pomegranate cluster covers just one-tenth the surface area of the individual particles inside it, a much smaller area is exposed to the electrolyte, thereby reducing the amount of gunk that forms to a manageable level.

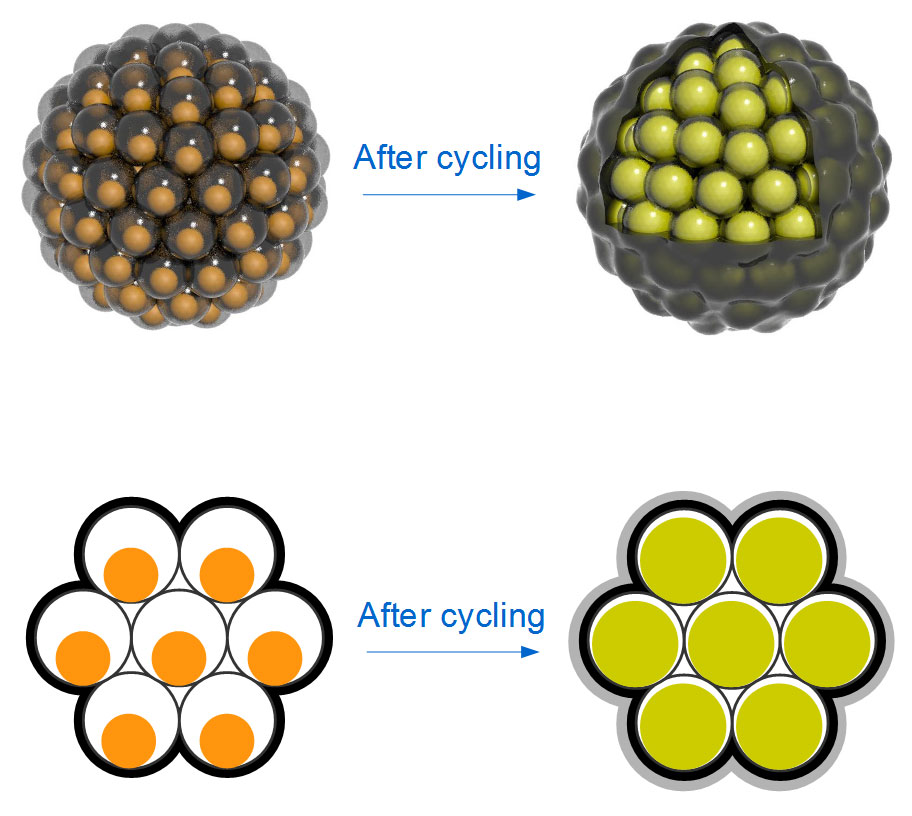
Top: Diagrammatic representation of Silicon nanoparticles clustered like seeds in a pomegranate.
Bottom: Silicon nanoparticles swell during battery charging to completely fill their yolk shells;
No space is wasted, and the shells stay intact.
Bottom: Silicon nanoparticles swell during battery charging to completely fill their yolk shells;
No space is wasted, and the shells stay intact.
Though the tests they have performed on the pomegranate batteries have shown results that are at par with other commercially available batteries, in order to begin mass production of this next-gen product, the researchers need to work on finding a cheaper source of silicon nanoparticles and simplifying the overall process. One of the researchers thinks that rice husks is one of the cheap alternative available today in bulk and it can be transformed into pure silicon nanoparticles relatively easily.
What do you think about the next generation of pomegranate inspired batteries being designed? Share your thoughts with us in comments below.
Source: #-Link-Snipped-#
0
