Molybdate, a new catalyst can turn CO2 emissions into useful polymers
Engineers at MIT have discovered a new catalyst called Molybdate (one molybdenum atom bonded with four oxygen atoms) that offers the much needed capability of trapping the carbon dioxide in air and converting it into useful polymers. The research is at primary stage and a lot more work is needed before efficient and economical processes can be developed to trap CO2; but if successful - it can help humans control the global warming to a greater extent. Christopher Cummins, professor of Chemistry at MIT says that Molybdate has the ability to fix the CO2 emitted by automobiles, factories and power plants - which continue to be the among the top sources of CO2. The aim behind the research is to develop carbon-neutral cycles for the cleaner energy and also minimize the pollution levels.
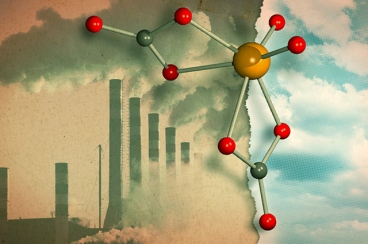
Credits: JOSE-LUIS OLIVARES/MIT; MOLYBDATE 3-D RENDERING BY LOANA KNOPF
The new reaction developed by chemists turns CO2 into carbonate ion (negatively charged) which reacts with silicon compound to create formate. Formate is one of the common starting material used in manufacturing of organic compounds. The process relies on molybdate - a catalyst that may be employed for larger scale industrial application. Molybdate is available abundantly and is stable in both air and water. No efforts were made until now to figure out how molybdate interacts with CO2.
Though Cummins and team have found promising results; they believe more research is needed to make the process useful for industries. They're now working on figuring out ways to trigger the reaction that results into creation of molybdate - which can be further used to catalyse other reactions.
The process of converting CO2 into various useful organic compounds is termed as 'Carbon Fixation'. The challenge lies in making carbon fixation economical and ready for mass adoption by the industries so that greater control can be achieved over the levels of CO2 in air. We wish Cummins and team all the best. Those interested in reading more about the research may head over to the source link below.
Source: #-Link-Snipped-#

Credits: JOSE-LUIS OLIVARES/MIT; MOLYBDATE 3-D RENDERING BY LOANA KNOPF
The new reaction developed by chemists turns CO2 into carbonate ion (negatively charged) which reacts with silicon compound to create formate. Formate is one of the common starting material used in manufacturing of organic compounds. The process relies on molybdate - a catalyst that may be employed for larger scale industrial application. Molybdate is available abundantly and is stable in both air and water. No efforts were made until now to figure out how molybdate interacts with CO2.
Though Cummins and team have found promising results; they believe more research is needed to make the process useful for industries. They're now working on figuring out ways to trigger the reaction that results into creation of molybdate - which can be further used to catalyse other reactions.
The process of converting CO2 into various useful organic compounds is termed as 'Carbon Fixation'. The challenge lies in making carbon fixation economical and ready for mass adoption by the industries so that greater control can be achieved over the levels of CO2 in air. We wish Cummins and team all the best. Those interested in reading more about the research may head over to the source link below.
Source: #-Link-Snipped-#
0
