Researchers Create Nano-Reactor For Producing Hydrogen Biofuel
Scientists from Indiana University have been successful in creating a highly proficient bio-material that catalyzes the production of hydrogen by incorporating a modified enzyme inside the virus.The efficient and renewable material can generate power from water, fueling cheap and efficient cars that run on water and can be reproduced en masse at room temperature using just a fermentation technology. The material is described to have a huge potential for clean and renewable energy applications that had been sought for too long in the scientific community.

Inside a capsid of virus (virus shell) enzyme hydrogenase, produced by two genes hyaA and hyaB, from the common bacteria Escherichia coli, are inserted. The capsid comes from the bacterial virus known as bacteriophage P22. Researchers took into account virus's ability to self-assemble myriad genetic building blocks and incorporated a very fragile and sensitive enzyme with the remarkable property of taking in protons and spitting out hydrogen gas. The resulting biomaterial, called "P22-Hyd," is not only more efficient than the unaltered enzyme but is potentially far less expensive and more environmentally friendly than other materials currently used to create fuel cells.
P22-Hyd both breaks the chemical bonds of water to create hydrogen and also works in reverse to recombine hydrogen and oxygen to generate power. The reaction runs both ways -- it can be used either as a hydrogen production catalyst or as a fuel cell catalyst; Prof.Trevor Douglas who led the study was quoted as saying.
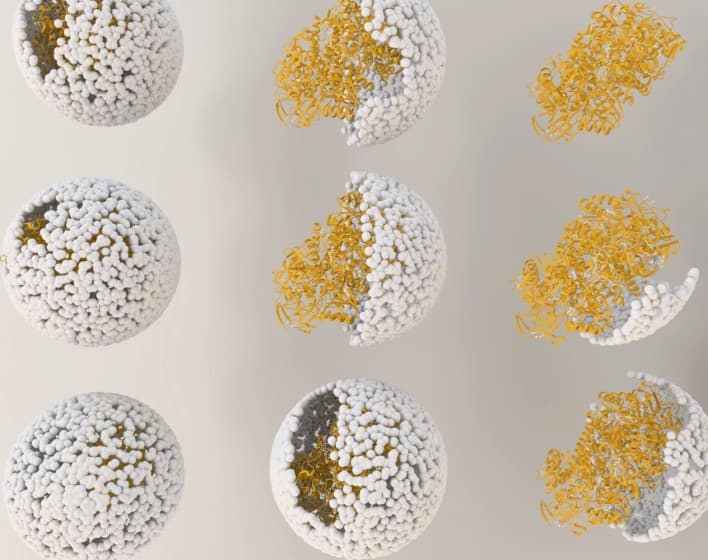
(Illustration showing the release of NiFe-hydrogenase from inside the virus shell, or "capsid," of bacteriophage P22. )
Out of the three naturally occurring forms of hydrogenase (di-iron (FeFe)-, iron-only (Fe-only)- and nickel-iron (NiFe)-hydrogenase) the last one was selected for the new material due to its ability to easily integrate into biomaterials, tolerate exposure to oxygen and retention of the ability to catalyze at room temperature. In its unaltered form however it posed considerable challenges for production of biofuels as it was highly susceptible to destruction from chemicals in the environment and broke down at higher temperatures, thereby rendering it unsuited for commercial production.
The pioneering work could help produce green clean fuels and tackle the glaring energy problem faced at present with great implications in near future.
For researchers investigating ways to incorporate the material into a solar-powered system is the next step of progression.
The research was published in the journal #-Link-Snipped-#
Source:#-Link-Snipped-#

Inside a capsid of virus (virus shell) enzyme hydrogenase, produced by two genes hyaA and hyaB, from the common bacteria Escherichia coli, are inserted. The capsid comes from the bacterial virus known as bacteriophage P22. Researchers took into account virus's ability to self-assemble myriad genetic building blocks and incorporated a very fragile and sensitive enzyme with the remarkable property of taking in protons and spitting out hydrogen gas. The resulting biomaterial, called "P22-Hyd," is not only more efficient than the unaltered enzyme but is potentially far less expensive and more environmentally friendly than other materials currently used to create fuel cells.
P22-Hyd both breaks the chemical bonds of water to create hydrogen and also works in reverse to recombine hydrogen and oxygen to generate power. The reaction runs both ways -- it can be used either as a hydrogen production catalyst or as a fuel cell catalyst; Prof.Trevor Douglas who led the study was quoted as saying.

(Illustration showing the release of NiFe-hydrogenase from inside the virus shell, or "capsid," of bacteriophage P22. )
Out of the three naturally occurring forms of hydrogenase (di-iron (FeFe)-, iron-only (Fe-only)- and nickel-iron (NiFe)-hydrogenase) the last one was selected for the new material due to its ability to easily integrate into biomaterials, tolerate exposure to oxygen and retention of the ability to catalyze at room temperature. In its unaltered form however it posed considerable challenges for production of biofuels as it was highly susceptible to destruction from chemicals in the environment and broke down at higher temperatures, thereby rendering it unsuited for commercial production.
The pioneering work could help produce green clean fuels and tackle the glaring energy problem faced at present with great implications in near future.
For researchers investigating ways to incorporate the material into a solar-powered system is the next step of progression.
The research was published in the journal #-Link-Snipped-#
Source:#-Link-Snipped-#
Replies
You are reading an archived discussion.
Related Posts
Hey, for a project at school (with solidworks) , I am making an angle that holds two boards at an angle of ninety degrees under pressure.
(sketch Included)
How can...
At the CES 2016 event today, Lenovo and Google jointly announced that a new smartphone which leverages Project Tango is on the cards. Google had been working on Project Tango...
The global networking products manufacturer TP-Link has its own smartphone brand called 'Neffos' and now it features three new smartphones, namely - C5L, C5 and C5 Max. Running atop Android...
Intel has recently launched its new developer phone , the 'Intel RealSense Smartphone' at the CES 2016 held in Las Vegas, Nevada. Priced at $399, it is already up for...
Researchers from China have created a new material that can convert atmospheric carbon dioxide into clean fuel. The material is made of partially oxidized cobalt layers, and carries out the...
