Interconnected SnO2 Nanoparticles To Increase Storage Capacity Of Lithium-ion Batteries
Now-a-days, for our daily requirements we need long lasting batteries. The consumer electronics for everyday use such as smartphones, digital cameras, MP3 players, tablets, et al works on batteries. Most of us don’t want to spend hours while waiting for these batteries to get charged. In order to fulfill the demand of charging the batteries in minutes, the researchers from Purdue University have designed a new electrode for lithium-ion (Li-ion) batteries. They have replaced the traditional graphite electrode with a network of tin-oxide (SnO2) nanoparticles.
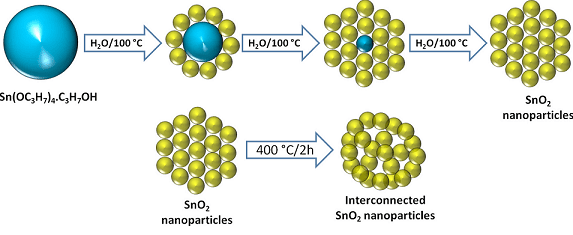
The diagrammatic representation of the concept of new electrode design for Li-ion batteries
For improving the Li-ion storage electrochemical performance, the anode of the battery is replaced by porous interconnected SnO2 nanoparticles. Theoretically, maximum storage capacity of graphite is 372 milliamp hours per gram (mAh g−1) while the tin-oxide based anode has almost twice the theoretical charging capacity of graphite. According to Vilas Pol, an associate professor of chemical engineering at Purdue University, the storage capacity of graphite is inadequate and is impeding the remarkable advances in battery technology.
While performing the experiment with tin-oxide based anode, the team found that the experimental anode can be charged in 30 minutes. Yet, have a capacity of 430 milliamp hours per gram which is more when compared to the theoretical maximum capacity for graphite when charged slowly over 10 hours. The tin oxide nanoparticles assemble themselves spontaneously into a network containing pores when heated at 400 degrees Celsius. Due to the pores, the material is able to expand and contract during the charge-discharge battery cycle.
This new anode would have a practical application in commercial manufacturing because it can be made by adding the tin alkoxide into boiling water. Pol said in a statement that they have not used any sophisticated chemistry; they have only boiled the solid precursor compound- tin alkoxide in water. This material is analogous to titanium alkoxides as they are cost-effective and widely available.
At present the research is at the nascent stage and the researchers will carry out the research further by testing the battery's s capability to work during many charge-discharge cycles in fully functioning batteries.
The research was published in November in the journal #-Link-Snipped-#.
Source: <a href="https://www.purdue.edu/newsroom/releases/2014/Q4/nanoparticle-network-could-bring-fast-charging-batteries.html" target="_blank" rel="nofollow noopener noreferrer">Nanoparticle network could bring fast-charging batteries - Purdue University</a>
The diagrammatic representation of the concept of new electrode design for Li-ion batteries
For improving the Li-ion storage electrochemical performance, the anode of the battery is replaced by porous interconnected SnO2 nanoparticles. Theoretically, maximum storage capacity of graphite is 372 milliamp hours per gram (mAh g−1) while the tin-oxide based anode has almost twice the theoretical charging capacity of graphite. According to Vilas Pol, an associate professor of chemical engineering at Purdue University, the storage capacity of graphite is inadequate and is impeding the remarkable advances in battery technology.
While performing the experiment with tin-oxide based anode, the team found that the experimental anode can be charged in 30 minutes. Yet, have a capacity of 430 milliamp hours per gram which is more when compared to the theoretical maximum capacity for graphite when charged slowly over 10 hours. The tin oxide nanoparticles assemble themselves spontaneously into a network containing pores when heated at 400 degrees Celsius. Due to the pores, the material is able to expand and contract during the charge-discharge battery cycle.
This new anode would have a practical application in commercial manufacturing because it can be made by adding the tin alkoxide into boiling water. Pol said in a statement that they have not used any sophisticated chemistry; they have only boiled the solid precursor compound- tin alkoxide in water. This material is analogous to titanium alkoxides as they are cost-effective and widely available.
At present the research is at the nascent stage and the researchers will carry out the research further by testing the battery's s capability to work during many charge-discharge cycles in fully functioning batteries.
The research was published in November in the journal #-Link-Snipped-#.
Source: <a href="https://www.purdue.edu/newsroom/releases/2014/Q4/nanoparticle-network-could-bring-fast-charging-batteries.html" target="_blank" rel="nofollow noopener noreferrer">Nanoparticle network could bring fast-charging batteries - Purdue University</a>
0
