For The First Time, Researchers Design Advanced Set-Up to throw light on Van Der Waal's Force
If we flip through the pages of any standard chemistry book, we would surely find a section in physical chemistry concerning chemical bonds, where we might encounter a quick note related to Van Der Waal’s force, underlined or marked with an asterisk. If we recall our chemistry lessons, we would remember that the Van Der Waal’s force is a weak intermoulecular or interatomic force that could be sustained if covalent or ionic bonds aren’t taking place. Though relatively weak, it could be divided in two parts, the first being the weak London Dispersion Forces and the much stronger Dipole-Dipole forces.
Although the impact is apparently less relevant with respect to the different types of chemical bonding, it is one of the foundations, based on which supramolecular chemistry, structural biology, polymer science, nanotechnology and condensed matter physics have grown. Such basic concepts however were subjected to information gaps and experimental support, which in turn generated missing links. A group of researchers from both the Swiss Nanoscience Institute and the University of Basel have made an astounding discovery by calculating, for the first time, small scale Van Der Waal's forces between individual atoms, thereby proving the speculation.
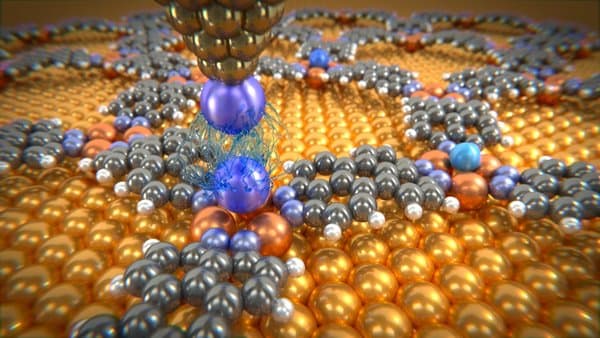
Rare gas atoms deposited on molecular network are investigated with a probing tip
Talking about the experimental set up, the team fed individual noble gas atoms inside a predesigned molecular network, where each one was fitted into their egg-shell. Then they were analyzed using a Xenon atom in terms of the reaction response with respective atoms, which was placed at the peak of an atomic force microscope. The ideal temperature range to carry out the experiment was at -300 degree Centigrade. As theorized, the setup unanimously proved that the force between two atoms is directly dependent on the distance but some sets had shown gross variance, the experimental results being significantly larger than the expected value.
The Van Der Waal’s force is a manifestation of the position changing phenomenon of electrons. Modern physics predicts that outside the nucleus of an atom, a dense electron cloud exists where an electron can be present “anywhereâ€. While an electron changes its place,
dipoles are rarely formed and a redistribution is further imposed on neighbouring molecules. If more than one dipoles are formed in neighbor atoms, a dipole-dipole interaction takes place generating a weak tension scientifically dubbed as Van Der Waal’s force. In spite of having a short life and being the weakest bond formed in nature, it proliferates as other forces coalesce as a single one and produce an affect large enough to be perceived using a macro-scaled microscope.
Quiet expectedly, the graphs almost matched with the theory which said, that with the increment of distance between two molecules, the force goes down sharply, however some tweaks were identified which might reveal a new side of physics, which we are not at all aware of. The results were published in the Nature Communication journal.
Source: #-Link-Snipped-#
Although the impact is apparently less relevant with respect to the different types of chemical bonding, it is one of the foundations, based on which supramolecular chemistry, structural biology, polymer science, nanotechnology and condensed matter physics have grown. Such basic concepts however were subjected to information gaps and experimental support, which in turn generated missing links. A group of researchers from both the Swiss Nanoscience Institute and the University of Basel have made an astounding discovery by calculating, for the first time, small scale Van Der Waal's forces between individual atoms, thereby proving the speculation.

Rare gas atoms deposited on molecular network are investigated with a probing tip
Talking about the experimental set up, the team fed individual noble gas atoms inside a predesigned molecular network, where each one was fitted into their egg-shell. Then they were analyzed using a Xenon atom in terms of the reaction response with respective atoms, which was placed at the peak of an atomic force microscope. The ideal temperature range to carry out the experiment was at -300 degree Centigrade. As theorized, the setup unanimously proved that the force between two atoms is directly dependent on the distance but some sets had shown gross variance, the experimental results being significantly larger than the expected value.
The Van Der Waal’s force is a manifestation of the position changing phenomenon of electrons. Modern physics predicts that outside the nucleus of an atom, a dense electron cloud exists where an electron can be present “anywhereâ€. While an electron changes its place,
dipoles are rarely formed and a redistribution is further imposed on neighbouring molecules. If more than one dipoles are formed in neighbor atoms, a dipole-dipole interaction takes place generating a weak tension scientifically dubbed as Van Der Waal’s force. In spite of having a short life and being the weakest bond formed in nature, it proliferates as other forces coalesce as a single one and produce an affect large enough to be perceived using a macro-scaled microscope.
Quiet expectedly, the graphs almost matched with the theory which said, that with the increment of distance between two molecules, the force goes down sharply, however some tweaks were identified which might reveal a new side of physics, which we are not at all aware of. The results were published in the Nature Communication journal.
Source: #-Link-Snipped-#
0
